The success rate of stem cell therapy for Parkinson’s disease is a topic of ongoing research and debate, with clinical trials showing promising results but also highlighting the need for further studies and refinement of techniques.
Stem cell therapy involves using stem cells to repair or replace damaged cells in the brain, offering potential for treating neurodegenerative diseases like Parkinson’s. However, the effectiveness of this approach and the factors influencing its success require careful examination.
Overview of Stem Cell Therapy for Parkinson’s Disease
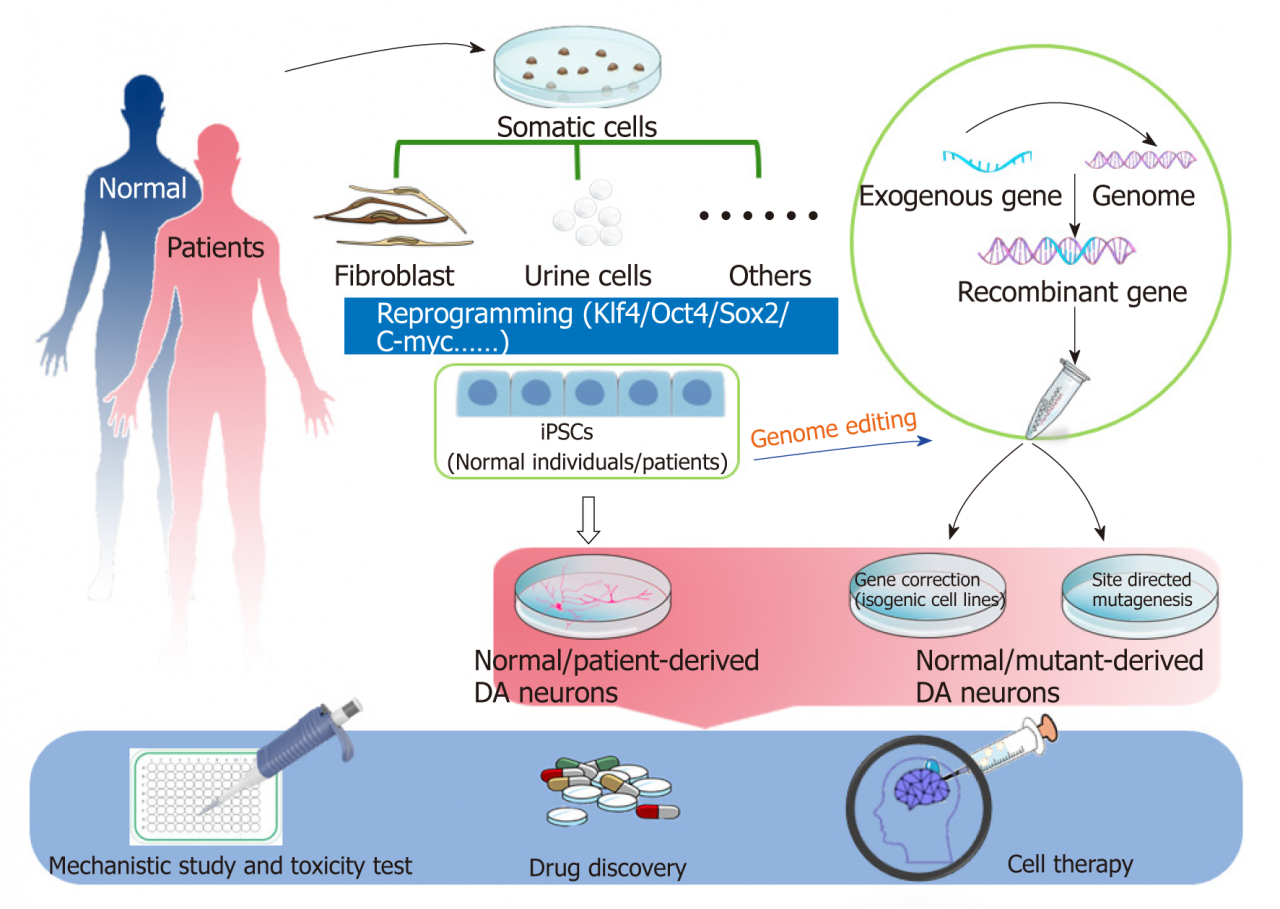
Stem cell therapy is a promising approach for treating Parkinson’s disease, a progressive neurological disorder characterized by the loss of dopamine-producing neurons in the brain. The goal of stem cell therapy is to replace or repair these lost neurons, restoring dopamine production and alleviating the symptoms of Parkinson’s disease.
There are two main types of stem cells used in stem cell therapy for Parkinson’s disease: embryonic stem cells and induced pluripotent stem cells (iPSCs). Embryonic stem cells are derived from human embryos, while iPSCs are created by reprogramming adult cells into a stem cell-like state.
Stem cell therapy for Parkinson’s disease has the potential to provide several benefits, including reducing motor symptoms, improving cognitive function, and slowing disease progression. However, there are also potential risks associated with stem cell therapy, such as the formation of tumors or immune rejection.
Clinical Trials and Success Rates: Success Rate Of Stem Cell Therapy For Parkinson’s Disease
Several clinical trials have been conducted to evaluate the safety and efficacy of stem cell therapy for Parkinson’s disease. The results of these trials have been mixed, with some studies showing promising results while others have reported limited benefits.
One of the most promising clinical trials was conducted by the Parkinson’s Institute and Clinical Center in Sunnyvale, California. This trial involved 18 patients with advanced Parkinson’s disease who were treated with stem cells derived from their own skin cells.
The results of the trial showed that the patients experienced significant improvements in their motor symptoms, with some patients even being able to reduce their medication dosage.
However, other clinical trials have reported less promising results. A study conducted by the Mayo Clinic in Rochester, Minnesota, found that stem cell therapy did not provide any significant benefits for patients with Parkinson’s disease. This study involved 10 patients who were treated with stem cells derived from human embryos.
The success rate of stem cell therapy for Parkinson’s disease is likely to vary depending on a number of factors, including the type of stem cells used, the stage of the disease, and the patient’s overall health.
Methods and Procedures
Stem cell therapy for Parkinson’s disease is typically performed using a surgical procedure called stereotactic surgery. This procedure involves using a computer-guided system to precisely deliver stem cells to the affected areas of the brain.
The surgical procedure typically takes several hours and is performed under general anesthesia. The patient is placed in a head frame, and a small incision is made in the scalp. A needle is then inserted into the brain, and the stem cells are injected through the needle.
After the surgery, the patient is typically monitored in the hospital for a few days. The patient may experience some pain or discomfort, but this can usually be managed with medication.
Patient Outcomes and Case Studies
The outcomes of stem cell therapy for Parkinson’s disease can vary from patient to patient. Some patients experience significant improvements in their symptoms, while others experience little to no benefit.
One patient who has experienced significant benefits from stem cell therapy is a man named David Parkinson. David was diagnosed with Parkinson’s disease in 2003, and his symptoms gradually worsened over the years. In 2016, David underwent stem cell therapy at the Parkinson’s Institute and Clinical Center in Sunnyvale, California.
The success rate of stem cell therapy for Parkinson’s disease remains a topic of ongoing research, with promising results in clinical trials. Meanwhile, in the world of football, wrexham transfer rumours continue to swirl, with speculation mounting over potential signings and departures.
Despite the excitement surrounding these rumours, the focus remains on the medical advancements in stem cell therapy, which offer hope for improving the lives of those affected by Parkinson’s disease.
After the surgery, David’s symptoms improved dramatically. He was able to reduce his medication dosage, and he experienced significant improvements in his motor skills. David is now able to walk, talk, and eat without assistance.
Ethical Considerations and Future Directions
Stem cell therapy for Parkinson’s disease raises a number of ethical considerations. One concern is the use of human embryos in stem cell research. Some people believe that it is unethical to use human embryos for research purposes, as this could potentially lead to the destruction of human life.
Another ethical concern is the potential for stem cell therapy to be used for non-therapeutic purposes. For example, stem cell therapy could potentially be used to enhance human performance or to create designer babies.
Despite these ethical concerns, stem cell therapy remains a promising approach for treating Parkinson’s disease. Further research is needed to evaluate the safety and efficacy of stem cell therapy, and to address the ethical concerns that it raises.
Outcome Summary

In conclusion, while stem cell therapy holds great promise for treating Parkinson’s disease, ongoing research and advancements in techniques are necessary to improve success rates and address challenges. Further studies will help optimize patient selection, delivery methods, and post-operative care to maximize the benefits of this innovative treatment.